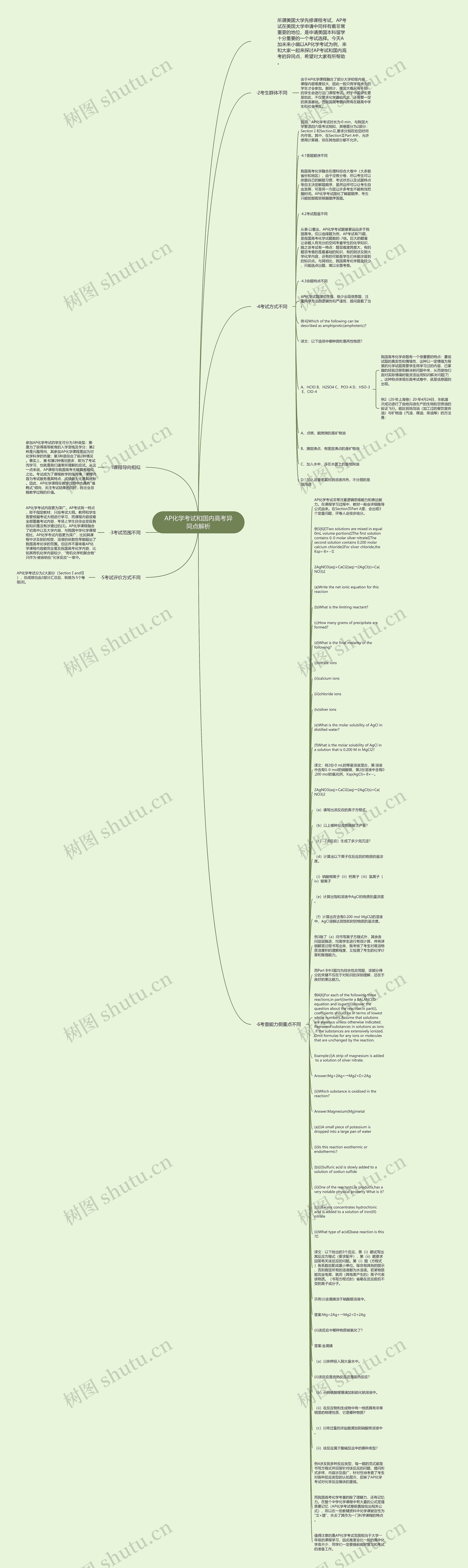
AP化学考试非常注重逻辑思维能力和表达能力。在课程学习过程中,教材一般会详细推导公式由来。在SectionⅡPart A里,会出现3个定量问题,评卷人会按步给分。
例3[6]Two solutions are mixed in equal·0mL volume portionsThe first solution contains 0.·0 molar silver nitrateThe second solution contains 0.200 molar calcium chlorideFor silver chloride,the Ksp=·8×·-·
2AgNO3(aq)+CaCl2(aq)→2AgCl(s)+Ca(NO3)2
(a)Write the net ionic equation for this reaction
(b)What is the limiting reactant?
(c)How many grams of precipitate are formed?
(d)What is the final molarity of the following?
(e)What is the molar solubility of AgCl in distilled water?
(f)What is the molar solubility of AgCl in a solution that is 0.200 M in MgCl2?
译文:将2份·0 mL的等量溶液混合,第·溶液中含有0.·0 mol的硝酸银,第2份溶液中含有0.200 mol的氯化钙,Ksp(AgCl)=·8×·-·。
2AgNO3(aq)+CaCl2(aq)→2AgCl(s)+Ca(NO3)2
(i)硝酸根离子(ii)钙离子(iii)氯离子(iv)银离子
(f)计算出在含有0.200 mol MgCl2的溶液中,AgCl溶解达到饱和时的物质的量浓度。
例3除了(a)问书写离子方程式外,其余各问层层推进,均需学生进行有效计算,并将详细解答过程书写出来,既考核了考生对难溶物质溶度积的理解程度,又检测了考生的化学计算和推理能力。
而Part B中3题均为综合性应用题,该部分得分的关键不仅在于对知识的深刻理解,还在于良好的表达能力。
例4[6]For each of the following three reactions,in part(i)write a BALANCED equation and in part(ii)answer the question about the reaction.In part(i),coefficients should be in terms of lowest whole numbers.Assume that solutions are aqueous unless otherwise indicated.Represent substances in solutions as ions if the substances are extensively ionized.Omit formulas for any ions or molecules that are unchanged by the reaction.
Example:(i)A strip of magnesium is added to a solution of silver nitrate.
(ii)Which substance is oxidized in the reaction?
Answer:Magnesium(Mg)metal
(a)(i)A small piece of potassium is dropped into a large pan of water
(ii)Is this reaction exothermic or endothermic?
(b)(i)Sulfuric acid is slowly added to a solution of sodiun sulfide
(ii)One of the reactants,or products,has a very notable physical property What is it?
(c)(i)Excess concentrates hydrochloric acid is added to a solution of iron(III)nitrate
(ii)What type of acidbase reaction is this?
译文:以下给出的3个反应,第(i)题试写出其反应方程式(要求配平),第(ii)题要求回答有关该反应的问题。第(i)题(方程式)各系数应配成最小单位。除非有其他的提示,否则假定所有的溶液都为水溶液。若某物质能完全电离,就用(其电离产生的)离子代表该物质。(书写方程式时)省略在反应前后不变的离子或分子。
(ii)在反应物和生成物中有一物质具有非常明显的物理性质,它是哪种物质?
例4涉及到多种反应类型,每一题的范式都是书写方程式并回答针对该反应的问题,提问形式多样,内容涉及面广,针对性地考查了考生对各种反应类型的认知层次,反映了AP化学考试对化学反应模块的重视。
而我国高考化学考查的除了理解力,还有记忆力。在整个中学化学课程中有大量的公式定理需要记忆(AP化学考试卷前直接给出相关公式),所以在一些教辅资料中化学课被定性为"文+理",失去了其作为一门科学课程的特点。
值得注意的是AP化学考试范围相当于大学一年级的课程学习,因此难度会比一般的高中化学高不少,同学们一定要提前做好复习和考试的准备工作。
 U633687664
U633687664
 小包卡麻麻
小包卡麻麻