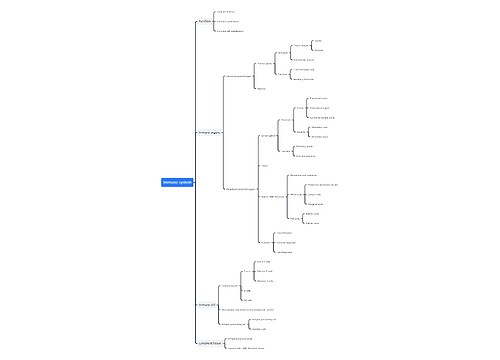
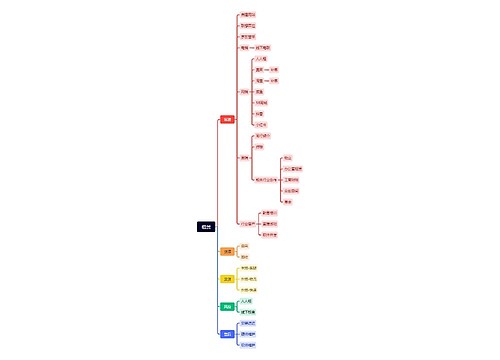
Immune regulation in glaucoma思维导图
U248871262
2023-10-26

青光眼
关键词
免疫调节
青光眼免疫力调节详解
树图思维导图提供《Immune regulation in glaucoma》在线思维导图免费制作,点击“编辑”按钮,可对《Immune regulation in glaucoma》进行在线思维导图编辑,本思维导图属于思维导图模板主题,文件编号是:8cc56a8a9482db7b70794386c85b1da0
思维导图大纲
相关思维导图模版
Immune regulation in glaucoma思维导图模板大纲
Content
#_Toc125451450Abstract 2
#_Toc125451451Introduction 2
#_Toc125451452Role of Cellular Immunity in The Immune Regulation of Glaucoma 3
#_Toc125451453The Role of Autoantibodies (AAbs) in The Immune Regulation of Glaucoma 4
#_Toc125451454Role of The Complement System in The Immune Regulation of Glaucoma 6
#_Toc125451455Role of Microglia in The Immune Regulation of Glaucoma 8
#_Toc125451456Conclusion 9
<a id="_Toc125451450"></a>Abstract
<a id="OLE_LINK1"></a>Glaucoma is a neurodegenerative disease characterized by progressive vision loss, which is primarily caused by retinal ganglion cell (RGC) dysfunction and death. The pathogenesis of this disease is complicated. In recent years, glaucoma has been proposed as a kind of autoimmune disease. It has been established that immune-mediated neurodegeneration contributes to glaucoma and plays a significant role in the disease's development. Immune cells, autoantibodies, the complement system, proinflammatory mediators, and microglia are all involved in the pathogenesis. Therefore, the main aim of this review was to summarize the current knowledge about changes in the above factors and their association with glaucoma, mainly focusing on the research direction of glaucomatous immune regulation in recent years, as a better understanding of its pathogenesis will greatly promote progress toward more effective methods for clinical treatment and improve the life quality of patients.
Keywords
<a id="_Toc125451451"></a>Introduction
The most frequent cause of irreversible blindness in the world, glaucoma is a chronic progressive optic neuropathy marked by dysfunction and death in the retinal nerve fiber layer and optic nerve head, which can result in a permanent loss of peripheral or central vision.[1] High intraocular pressure (IOP), advanced age, non-white race, a positive family history, and high myopia are additional significant risk factors for primary open-angle glaucoma (POAG), the most prevalent type of glaucoma. IOP is the primary modifiable risk factor, but the pathogenesis of glaucoma is still being understood and explored.[2] which can induce functional impairment and neurodegeneration of retinal ganglion cells (RGCs). Progressive loss of RGCs and damage to optic nerve fibers persist even after treatment is used to restore normal IOP, or in glaucoma patients with normal IOP. [3] As a result, the information presented above makes us consider the potential that glaucoma etiology may involve an additional immune-mediated mechanism. Dysregulation of the immune system may lead to neurodegeneration of glaucoma by interfering with the normal homeostasis system.
The eye is regarded as an immune-privileged site mainly based on the fact that the retina has a highly sensitive innate immune system. The blood-retinal barrier (BRB) serves as a physical barrier by which the retina defends itself against infections, pathogens and systemic inflammation. BRB is the first line of defense for the protection provided to the eye by the innate immune system. When pathogens break through BRB (i.e., when the eye is exposed to disease conditions like glaucoma), retinal cells can lessen immunopathology by initially suppressing a portion of the inflammatory response. Then, the antibodies will be transferred to the systemic immune system, resulting in a peripheral adaptive immunological response that can either induce immune response inhibition or gain tolerance. However, when pathogens or other risk factors interfere with cellular homeostasis and go beyond what these stresses can withstand, it can compromise retinal function.[4] Under retinal or subretinal immune detection, a series of immune responses, including innate and adaptive immune responses, are triggered in glaucoma patients, resulting in the activation of immune cells, the production of autoantibodies, the activation of the complement system, the activation of microglia, and ultimately the death of the RGCs. Hence, it is necessary to investigate the activity of the immune system in the pathogenesis of glaucoma, which will contribute to the development of therapeutic strategies that protect RGCs and maintain normal visual function. This review summarizes the latest research advances in immune mechanisms of glaucoma in the recent years, focusing on the role of immune factors in the pathogenesis of the disease.
<a id="_Toc125451452"></a>Role of Cellular Immunity in The Immune Regulation of Glaucoma
<a id="
The pathogenic role of T cells in glaucoma also involves regulatory T cells and helper T cells. There were no statistically significant differences in the distribution of IFN-c (Th1), IL-4 (Th2), or IL- 17A (Th17) subpopulations of peripheral blood T lymphocytes in glaucomatous samples, but all were detected at lower frequencies than in nonglaucomatous controls, as was the frequency of CD4 (or CD8)/CD25/FoxP3 Tregs. The proliferation and proinflammatory cytokine secretion of CD4 T lymphocytes as well as their inflammatory stimulation were increased.[11] Accordingly, the difference in frequency between CD4 Tregs and CD4 T lymphocytes may indicate an alteration in T cell-mediated immune homeostasis. However, in a different study, regulatory T cell levels were elevated in glaucoma patients, along with elevated levels of CD4+CD25+ lymphocytes. This may be because Tregs have the capacity to directly influence B cells that produce antibodies.[12] An imbalance in Th1/Th2 cytokine expression can be found in the iris of glaucomatous eyes, with increased expression of Th1 (IL-2 and IFN-γ) cytokines associated with neurological damage and decreased expression of Th2 (IL-6) cytokines associated with neuroprotection, which plays a role in regulating the immune microenvironment and damage to retinal ganglion cells in glaucoma patients.[13] However, as far as current studies are concerned, however, the analysis of T cells in glaucoma patients and experimental models is still scarce; only a few studies have focused on the presence of specific T lymphocyte subsets in the serum of glaucoma patients.
The numbers of total B cells, Antibody-Secreting Cells (ASC)/ plasmablasts, and naive cells in the peripheral blood of glaucomatous samples were significantly increased compared to those of healthy donors. And with the increase of clinical severity, pre-existing memory B cells can be reactivated and differentiated, leading to an increase in the frequency and number of atypical late memory B cells (CD27-IGD-DN subset).[14] Furthermore, in glaucoma patients, the accumulation of IgG autoantibodies on the retina is accompanied by an increase in the number of CD27+/IgG+ plasma cells.[15] Overall, increased total number of naive B cells, plasma cells and B cells indicate an overactive B cell-mediated immune response in patients with glaucoma, while the role of damage mechanisms mediated by B cells is limited.
Thus, the subpopulations of T/B cells that correlate with the clinical severity of the disease could suggest the potential of these subpopulations to be used as biomarkers for detecting disease progression. In addition to this, future studies are still further needed to expand the sample volume as well as to continue to detect changes in T/B cell subpopulations and changes in T/B cell-mediated immune homeostasis in retinal biopsies of glaucoma patients, to clarify the role of T/B cells in glaucoma pathogenesis and role of T/B cell homeostasis, to discover the link between altered T/B cell homeostasis and neurodegeneration in glaucoma, and whether biomarkers as autoimmune susceptibility can be identified from them, which could help to develop new treatments or interventions.
<a id="_Toc125451453"></a>The Role of Autoantibodies (AAbs) in The Immune Regulation of Glaucoma
<a id="OLE_LINK5"></a>Indeed, natural autoantibodies (AAbs) have neuroprotective properties. They are regulators that can be involved in a vast array of physiological processes, including immunomodulation, homeostasis, and repertoire selection, as well as anti-infection, transport, and the functional regulation of bioactive substances.[16,17] Neurodegenerative processes can be slowed down and the spread of neuronal damage induced by various risk factors can be mitigated by immune-protective processes. When the equilibrium of specific AAbs is disrupted, for example when protective autoantibodies are reduced, the regulatory function of these antibodies is altered When their neuroprotective effect is lost, they may become more sensitive to risk factors or become self-aggressive, promoting neurodegeneration. Hence, certain variations in AAbs might be considered as clinical indicators of autoimmune diseases. [18–20]
Some immunoregulatory mechanisms in glaucoma may be comparable to those seen in autoimmune diseases, and patients with glaucoma exhibit changes in anti-retinal and optic nerve antigen antibody concentrations and higher T-cell reactivity to retinal antigens.[11] The detection of antigens and autoantibodies that activate the immune response in the patient's serum and aqueous humour, despite the fact that the patterns of immunoreactivity in serum and aqueous humour are not significantly different,[21] which is due to the greater immune reactivity of the serum to retinal proteins and the glaucomatous alterations in protein expression that affect the antigenic characteristics of the proteins.[22] <a id="
There are other in vitro experiments that have identified a role for certain specific autoantibodies in glaucomatous neurodegeneration. One research, employing serological proteomic analysis (SERPA) to analyze the autoantibody profiling of glaucoma samples, found that VDAC2, CALD1 and PGAM1 AAbs were elevated in the sera of both POAG patients and early POAG patients. [27]As a result, there is a certain clinical value to the monitoring of VDAC2, CALD1, and PGAM1 AAbs in the early phase of POAG. Researchers compared the autoantigens of healthy and glaucomatous trabecular meshwork (TM) cell lines using a technique known as mass spectrometry-based antibody-mediated identification of autoantigens (MS - AMIDA). They discovered that 21 autoantigens were associated with POAG, and that the platelet-derived growth factor receptor beta (PDGFRB) pathway, which is involved in TM fibrosis, was particularly enriched in POAG-associated antigens.[28] In addition to this, patients with glaucoma had significantly higher autoantibody levels for the PDGFRB pathway proteins threonine-tRNA ligase (TARS), component 1 Q subcomponent-binding protein (C1QBP), and paraneoplastic antigen Ma2 (PNMA2).[28] <a id="OLE_LINK6"></a>Patients diagnosed with acute angle-closure glaucoma were shown to have significant variations in their levels of anti-HSP27 antibody, anti-TTLL12 antibody, and anti-NSE antibody, all of which were caused by IOP.[29] Anti-adaptor protein 1 complex subunit mu-1 antibodies and anti-SPRY domain-containing SOCS box protein 3 antibodies levels were found to be positively associated with persistent progression in patients with primary open-angle glaucoma combined with optic disc hemorrhage.[30] By lowering endoplasmic reticulum stress levels and promoting the migration of glutamine synthetase to the inner retinal layer, the gamma (γ)-synuclein and glial fibrillary acidic protein (GFAP) antibodies exhibit protective effects on RGCs.[31]
Immunoglobulin G (IgG) AAbs also play a role in the damage of glaucomatous RGCs , such as their deposition on glaucomatous retinas in an inflammatory environment. Glaucoma patients exhibit variations in the concentration of anti-retinal and optic nerve antigen antibodies. Peptides of the variable IgG domain showed significant changes in glaucoma patients, which demonstrated for the first time that structures of the variable region of antibodies, complementarity determining region (CDR), is closely related to glaucoma. [32]The presence of disease-specific variations in the complex spectrum of spontaneously occurring IgG AAbs in glaucoma patients' serum and atrial fluid, as well as the involvement of IgG AAbs in the damage and apoptosis of RGCs in an experimental autoimmune glaucoma model (EAG model), and the deposition of IgG autoantibodies with optic nerve and retinal degeneration seen in an intermittent monocular hypertension model, strongly indicate that IgG AAbs are involved in the pathogenesis of glaucoma and play an important role in causing damage to RGCs.[15,20,33,34]
<a id="_Hlk116852432"></a>Antibodies to heat shock proteins are involved in glaucomatous neurodegeneration. In actuality, heat shock proteins are immunogenic proteins with a high degree of evolutionary conservation. [35] On the retina, the HSPs family plays a crucial regulatory role in homeostasis, cellular defense, pathogenic immunological responses, and neurodegenerative processes.[36] They contribute to the denaturation of denatured proteins and play a role in antigen presentation to stimulate the innate and adaptive immune systems.[37,38] Hence, understanding the role of HSP in the pathogenesis of glaucoma is essential to our understanding of the neurodegeneration of glaucoma.
Using the EAG model, Wax et al. demonstrated that HSP27 and HSP60 can result in RGC loss.[39] In animal models of experimental IOP elevation, there is increased expression of HSP27, HSP60 and HSP72 as well as increased expression of TLR (Toll-like receptor family) 2, 3, 4 and characteristic TLR signaling cascade adapter proteins and kinases.[40] This suggests that TLR contributes to the activation of the glaucomatous immune system by HSP. High extracellular HSP concentrations activate the immune system by acting in two main ways.[41] On the one hand, it occurs through binding to TLRs such as TLR-2 and TLR-4. [42] On the other side, molecular mimicry is the cause of autoimmune and cross-immune reactions.[43] After immunization with S100 and HSP27, loss of RCGs, AII-free long-synaptic cells, cone bipolar cells and synaptic connections was observed in the EAG model, but without additional retinal damage, presumably because the two antigens inhibited each other.[44] Patients with glaucoma also show these high levels of antibodies to heat-shock proteins (HSPs).[41] Additionally proving HSP-specific T-cell responses in glaucoma patients, HSP27 was discovered to be an antigen in IOP elevation-induced T-cell responses that mediated RGC prolongation and axonal degeneration in glaucoma animal tests.[9] Meanwhile, the mechanism of glaucomatous neurodegeneration is mediated by T cells pre-sensitized by exposure to the commensal microbiota.[9] One reason for this may be that specific T cells directed against bacterial HSP, under the influence of molecular mimicry, cross-react with endogenous homologous HSP and thus cause autoimmune damage.
The qualitative characteristics of the antigens and AAbs involved in the autoimmune reactions of glaucoma and the specific conditions of the autoimmune modifications still need to be supplemented, which will greatly help to understand the changes of autoantibodies in patients with different severity of glaucoma.
<a id="_Toc125451454"></a>Role of The Complement System in The Immune Regulation of Glaucoma
The complement system, known to consist of more than 50 proteins, is part of the innate immune response and is regarded to be essential for the rapid recognition and clearance and killing of pathogens, apoptotic cells, and cell debris that have breached the host's protective barrier. It works primarily through liquid phase circulation and binding to the cell membrane, where antibodies may trigger an adaptive response by activating the complement system leading to the death of glaucomatous retinal stem cells.[45,46] The classical pathway, lectin pathway, and alternate pathway are all involved in complement system activation. As a result, one of the causes of glaucoma may be the simultaneous or different activation of all three pathways, resulting in a complement system imbalance.
Although markedly high IOP can lead to activation of the complement system, activation of the complement system can also be detected in the presence of moderately high IOP, accompanied by loss of RGCs, which indicates the involvement of the immune system.[47] C3 is the central complement component and convergence point for all three pathways of the complement system. In a study, plasma complement and immunoglobulin levels were compared between patients with various types of glaucoma and healthy controls using the case-control method. The results suggest that in patients with acute primary angle-closure glaucoma (APACG), elevated IOP may be caused by the activation of the complement and autoimmune system, and that the upregulation of C3 through the classical combined alternative pathway may be the key to the damage during the acute episode of glaucoma. And plasma IgA, IgG and IgM levels were also significantly increased in APACG patients. Therefore, the expression levels of plasma C3, IgA, IgG and IgM evaluate the indicators of POAG.[48] The early immune response following early IOP elevation in glaucomatous mice is activation of complement C3, and early deficiency of C3 increases the loss of RGCs, suggesting a role for C3 in protecting against early glaucomatous damage.[49] Patients with POAG have a pronounced increase in the C3a/C3 ratio, which is closely related to glaucoma progression. This increase can be seen in both the atrial fluid and serum. [50] In addition, dysregulation of the complement system is associated with neural regeneration. The degeneration of retinal ganglion cell axons and neurons is delayed by sustaining glaucomatous retinal production of CR-Crry, a C3 inhibitor that reduces complement C3 and downstream complement activation.[51] Research related to high-pressure glaucoma has revealed that a strong increase in IOP of approximately 30% can lead to the formation of C1q, C3 and membrane attack complexes (MAC), which activate the complement system.[52]
In the glaucoma model, it is obvious that the complement system is activated by the lectin pathway. And the activation of the complement cascade reaction and the formation of membrane attack complexes (MACs) could also explain why the second eye of unilateral glaucoma patients does not develop the disease. In addition, MAC labeling can be observed in the retina of human donors with advanced glaucoma, which may be associated with the degeneration of RGCs.[53] Sabrina Reinehr et al. demonstrated for the first time the presence of complement activation triggered mainly through the lectin pathway in the EAG model and that complement activation can lead to death and optic neuropathy in RGCs.[54] In addition, after immunizing rats with S100B using the EAG model, the following changes occurred in the retinal tissue: the mannose-binding lectin (MBL) was significantly increased at 3,7 and 14 days, NFκB was enhanced at 7 and 14 days, and C3 level was increased at 14 days.[55] These results suggest that NFκB may be triggered by MBL and cause an imbalance of the complement system, which subsequently leads to cell damage and apoptosis.
Inhibition of complement C1 is also thought to be a strategy for the treatment of glaucoma, as research has found that inhibition of C1 function and knockdown of C1qa in a high IOP model can reduce retinal ganglion cell death and optic nerve damage by protecting dendritic and synaptic structures.[56] It was also shown by intravitreal administration of the monoclonal antibody BB5.1, which binds to the complement factor C5, that the C5 antibody inhibits the activation of the complement system by inhibiting MAC formation, preventing the loss of retinal function as well as protecting some RGCs and retinal cone bipolar cells.[57] Proteomic analysis of the atrial water of POAG revealed a significant downregulation of proteins of the classical pathway of the complement system.[58] In general, the complement system can be activated through three pathways leading to the loss of RGCs and by targeting the complement pathway to increase and decrease complement proteins can provide certain optic nerve protection in glaucoma.
<a id="_Toc125451455"></a>Role of Microglia in The Immune Regulation of Glaucoma
Microglia, which are tissue-resident macrophages of the central nervous system (CNS) and characterized by small cell bodies, ramified thin and spines surrounding blood vessels, play an important role in fighting infection, removing cell debris, and maintaining tissue homeostasis.[59] Microglia are closely related to the blood-brain barrier and are present in the retina, where they contribute to local immune responses and immune surveillance through proliferation, activation, and migration.[60] They attack the main carriers of ocular antigens, RGCs, and optic nerve fibers, which release death signals when damaged, leading to microglia recruitment in the retina. [61] Retinal glial cells interact with other cells in the glaucoma-associated retina, including RGCs, in<a id="_Hlk119870808"></a> a subtle relationship to execute their functions.[62]
The degree of microglia activation correlates with the degree of glaucomatous axonal degeneration, [63] and this microglia activation correlates with glaucomatous optic nerve axonal degeneration similarly to other degenerative diseases.[15] In the retina of mice inoculated with a retinal ganglion cell layer homogenate, increased levels of reactive gliosis, particularly Iba1+ microglia, were identified in the EAG model.[34] However, it was also discovered that inhibition of the complement system had no downregulatory impact on microglia in the EAG model, probably because inhibition of the complement system affected the cascade at later stages, while microglia were not affected.[57] In fact, more activated microglia were observed when complement inhibition was performed with the C5 antibody, [57] so this is one of the reasons why complement inhibition preserves some RGCs but not all of them. Microglia activation in the damaged retina can also be found in the previously mentioned monocular intermittent high IOP model.[64] Actually, activated microglia can trigger innate and adaptive immune responses by releasing and maintaining the secretion of pro-inflammatory mediators, leading to the loss of RGCs and deposition of IgG AAbs on the retina in patients with glaucoma.[15,63]
One study that used either S100 or ONA for immunization came to the conclusion that RGC loss and damage coincided with the initial microglial cell response.[65] NFκB may be able to control microglial migration to the site of injury through MBL triggering, resulting in the loss of RGCs.[66] The relationship between microglia and RGC depletion was further elucidated in a later experiment. Following intravitreal administration of S100B, there is a phenomenon where MBL increases at 3, 7, and 14 days, NF-KB content increases at 7, and 14 days[55], and microglia numbers increase at 14, along with a decrease of RGCs[67].
In addition, other factors act by affecting microglia. Alterations in the purinergic system can regulate the function of many microglia. High IOP circumstances associated with glaucoma may affect the purinergic systems of microglia and interfere with their ability to function, altering the purinergic system of glaucoma as a whole.[68] We have previously mentioned the presence of metastatic T cells in the spleen and retinal ganglion cell layer of mice with glaucoma. In reality, metastatic T cells can interact directly with activated tissue microglia and produce potentially damaging substances that accelerate the loss of RGCs.[8] Stimulation of activation of optic microglia with lipopolysaccharide had no significant effect on optic nerve damage, suggesting that microglia activation may be secondary to chronic neurodegeneration in glaucoma.[69] Contralateral eye involvement can also be found in mice with experimental glaucoma, and one reason can be explained by the activation of microglia.[70] In conclusion, whether microglia activation is a contributing factor to glaucoma or a consequence of glaucoma onset is undetermined and still needs to be explored in more experiments; what we can determine is that microglia activation is an integral part of glaucoma pathogenesis.
<a id="_Toc125451456"></a>Conclusion
Overall, the etiology of glaucoma is constantly being updated, with more and more studies confirming that immune-mediated neurodegeneration plays a crucial role. The development of RGC mortality and retinal degeneration in glaucoma patients is the result of interactions and changes in multiple immunological variables, leading to immune homeostasis imbalance. These changes include immune cell activation, autoantibody formation, complement system activation, and microglial activation. Understanding these changes in immune components can aid in the identification of new glaucoma treatment targets. When appropriate, targeted blockage or promotion of the expression of certain immune components is used to maintain immune privilege in the degenerating retina, such as when researching new glaucoma medicines, eye drops, or intravitreal injections of immune component inhibitors or enhancers. Currently, glaucoma is mostly treated clinically with medicines or glaucoma surgery to reduce IOP. However, there are no conclusive clinical findings indicating immunomodulation is beneficial for glaucoma patients. Consequently, there is still a need to expand the sample size of clinical studies in glaucoma and to determine the specificity of altered immune components and identify biomarkers of glaucoma, which would provide a more comprehensive understanding of the mechanisms of the immune response in glaucoma and better treatment options for glaucoma and reduce or prevent the adverse effects of glaucoma.
References
[1] STEIN J D, KHAWAJA A P, WEIZER J S. Glaucoma in adults—screening, diagnosis, and management: a review[J]. JAMA, 2021, 325(2): 164. DOI:10.1001/jama.2020.21899.
[2] JONAS J B, AUNG T, BOURNE R R, et al. Glaucoma[J]. The Lancet, 2017, 390(10108): 2183–2193. DOI:10.1016/S0140-6736(17)31469-1.
[3] DANFORD I D, VERKUIL L D, CHOI D J, et al. Characterizing the “poagome”: a bioinformatics-driven approach to primary open-angle glaucoma[J]. Progress in Retinal and Eye Research, 2017, 58: 89–114. DOI:10.1016/j.preteyeres.2017.02.001.
[4] CHEN M, LUO C, ZHAO J, et al. Immune regulation in the aging retina[J]. Progress in Retinal and Eye Research, 2019, 69: 159–172. DOI:10.1016/j.preteyeres.2018.10.003.
[5] BELL K, UND HOHENSTEIN-BLAUL N von T, TEISTER J, et al. Modulation of the immune system for the treatment of glaucoma[J]. Current Neuropharmacology, 2018, 16(7): 942–958. DOI:10.2174/1570159X15666170720094529.
[6] KUNKL M, FRASCOLLA S, AMORMINO C, et al. T helper cells: the modulators of inflammation in multiple sclerosis[J]. Cells, 2020, 9(2): E482. DOI:10.3390/cells9020482.
[7] LINDESTAM ARLEHAMN C S, GARRETTI F, SULZER D, et al. Roles for the adaptive immune system in parkinson’s and alzheimer’s diseases[J]. Current Opinion in Immunology, 2019, 59: 115–120. DOI:10.1016/j.coi.2019.07.004.
[8] GRAMLICH O W, DING Q J, ZHU W, et al. Adoptive transfer of immune cells from glaucomatous mice provokes retinal ganglion cell loss in recipients[J]. Acta Neuropathologica Communications, 2015, 3: 56. DOI:10.1186/s40478-015-0234-y.
[9] CHEN H, CHO K-S, VU T H K, et al. Commensal microflora-induced t cell responses mediate progressive neurodegeneration in glaucoma[J]. Nature Communications, 2018, 9(1): 3209. DOI:10.1038/s41467-018-05681-9.
[10] GRAMLICH O W, GODWIN C R, HEUSS N D, et al. T and b lymphocyte deficiency in rag1-/- mice reduces retinal ganglion cell loss in experimental glaucoma[J]. Investigative Ophthalmology & Visual Science, 2020, 61(14): 18. DOI:10.1167/iovs.61.14.18.
[11] YANG X, ZENG Q, GÖKTAS E, et al. T-lymphocyte subset distribution and activity in patients with glaucoma[J]. Investigative Ophthalmology & Visual Science, 2019, 60(4): 877–888. DOI:10.1167/iovs.18-26129.
[12] BELL K, HOLZ A, LUDWIG K, et al. Elevated regulatory t cell levels in glaucoma patients in comparison to healthy controls[J]. Current Eye Research, 2017, 42(4): 562–567. DOI:10.1080/02713683.2016.1205629.
[13] WONG M, HUANG P, LI W, et al. T-helper1/t-helper2 cytokine imbalance in the iris of patients with glaucoma[J]. PloS One, 2015, 10(3): e0122184. DOI:10.1371/journal.pone.0122184.
[14] YU L, CHEN Y, XU X, et al. Alterations in peripheral b cell subsets correlate with the disease severity of human glaucoma[J]. Journal of Inflammation Research, 2021, 14: 4827–4838. DOI:10.2147/JIR.S329084.
[15] GRAMLICH O W, BECK S, VON THUN UND HOHENSTEIN-BLAUL N, et al. Enhanced insight into the autoimmune component of glaucoma: igg autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina[J]. PloS One, 2013, 8(2): e57557. DOI:10.1371/journal.pone.0057557.
[16] SCHWARTZ M. Physiological approaches to neuroprotection: boosting of protective autoimmunity[J]. Survey of Ophthalmology, 2001, 45: S256–S260. DOI:10.1016/S0039-6257(01)00208-9.
[17] SHOENFELD Y, TOUBI E. Protective autoantibodies: role in homeostasis, clinical importance, and therapeutic potential[J]. Arthritis & Rheumatism, 2005, 52(9): 2599–2606. DOI:10.1002/art.21252.
[18] JOACHIM S C, PFEIFFER N, GRUS F H. Autoantibodies in patients with glaucoma: a comparison of igg serum antibodies against retinal, optic nerve, and optic nerve head antigens[J]. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie, 2005, 243(8): 817–823. DOI:10.1007/s00417-004-1094-5.
[19] JOACHIM S C, GRUS F H, PFEIFFER N. Analysis of autoantibody repertoires in sera of patients with glaucoma[J]. European Journal of Ophthalmology, 2003, 13(9–10): 752–758. DOI:10.1177/1120672103013009-1003.
[20] GRUS F H, JOACHIM S C, HOFFMANN E M, et al. Complex autoantibody repertoires in patients with glaucoma[J]. Molecular Vision, 2004, 10: 132–137.
[21] BOEHM N, WOLTERS D, THIEL U, et al. New insights into autoantibody profiles from immune privileged sites in the eye: a glaucoma study[J]. Brain, Behavior, and Immunity, 2012, 26(1): 96–102. DOI:10.1016/j.bbi.2011.07.241.
[22] TEZEL G, THORNTON I L, TONG M G, et al. Immunoproteomic analysis of potential serum biomarker candidates in human glaucoma[J]. Investigative Opthalmology & Visual Science, 2012, 53(13): 8222. DOI:10.1167/iovs.12-10076.
[23] GRUS F H, JOACHIM S C, BRUNS K, et al. Serum autoantibodies to alpha-fodrin are present in glaucoma patients from germany and the united states[J]. Investigative Ophthalmology & Visual Science, 2006, 47(3): 968–976. DOI:10.1167/iovs.05-0685.
[24] JOACHIM S C, REICHELT J, BERNEISER S, et al. Sera of glaucoma patients show autoantibodies against myelin basic protein and complex autoantibody profiles against human optic nerve antigens[J]. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie, 2008, 246(4): 573–580. DOI:10.1007/s00417-007-0737-8.
[25] JOACHIM S C, BRUNS K, LACKNER K J, et al. Antibodies to alpha b-crystallin, vimentin, and heat shock protein 70 in aqueous humor of patients with normal tension glaucoma and igg antibody patterns against retinal antigen in aqueous humor[J]. Current Eye Research, 2007, 32(6): 501–509. DOI:10.1080/02713680701375183.
[26] BELL K, WILDING C, FUNKE S, et al. Protective effect of 14-3-3 antibodies on stressed neuroretinal cells via the mitochondrial apoptosis pathway[J]. BMC Ophthalmology, 2015, 15: 64. DOI:10.1186/s12886-015-0044-9.
[27] BEUTGEN V M, PERUMAL N, PFEIFFER N, et al. Autoantibody biomarker discovery in primary open angle glaucoma using serological proteome analysis (serpa)[J]. Frontiers in Immunology, 2019, 10: 381. DOI:10.3389/fimmu.2019.00381.
[28] BEUTGEN V M, SCHMELTER C, PFEIFFER N, et al. Autoantigens in the trabecular meshwork and glaucoma-specific alterations in the natural autoantibody repertoire[J]. Clinical & Translational Immunology, 2020, 9(3): e01101. DOI:10.1002/cti2.1101.
[29] LORENZ K, BECK S, KEILANI M M, et al. Course of serum autoantibodies in patients after acute angle-closure glaucoma attack[J]. Clinical & Experimental Ophthalmology, 2017, 45(3): 280–287. DOI:10.1111/ceo.12864.
[30] LORENZ K, BECK S, KEILANI M M, et al. Longitudinal analysis of serum autoantibody-reactivities in patients with primary open angle glaucoma and optic disc hemorrhage[J]. PloS One, 2016, 11(12): e0166813. DOI:10.1371/journal.pone.0166813.
[31] BELL K, WILDING C, FUNKE S, et al. Neuroprotective effects of antibodies on retinal ganglion cells in an adolescent retina organ culture[J]. Journal of Neurochemistry, 2016, 139(2): 256–269. DOI:10.1111/jnc.13765.
[32] SCHMELTER C, PERUMAL N, FUNKE S, et al. Peptides of the variable igg domain as potential biomarker candidates in primary open-angle glaucoma (poag)[J]. Human Molecular Genetics, 2017, 26(22): 4451–4464. DOI:10.1093/hmg/ddx332.
[33] JOACHIM S C, GRUS F H, KRAFT D, et al. Complex antibody profile changes in an experimental autoimmune glaucoma animal model[J]. Investigative Ophthalmology & Visual Science, 2009, 50(10): 4734–4742. DOI:10.1167/iovs.08-3144.
[34] JOACHIM S C, GRAMLICH O W, LASPAS P, et al. Retinal ganglion cell loss is accompanied by antibody depositions and increased levels of microglia after immunization with retinal antigens[J]. PloS One, 2012, 7(7): e40616. DOI:10.1371/journal.pone.0040616.
[35] LINDQUIST S, CRAIG E A. The heat-shock proteins[J]. Annual Review of Genetics, 1988, 22: 631–677. DOI:10.1146/annurev.ge.22.120188.003215.
[36] JIANG S, KAMETANI M, CHEN D F. Adaptive immunity: new aspects of pathogenesis underlying neurodegeneration in glaucoma and optic neuropathy[J]. Frontiers in Immunology, 2020, 11: 65. DOI:10.3389/fimmu.2020.00065.
[37] BINDER R J. Functions of heat shock proteins in pathways of the innate and adaptive immune system[J]. The Journal of Immunology, 2014, 193(12): 5765–5771. DOI:10.4049/jimmunol.1401417.
[38] SRIVASTAVA P. Roles of heat-shock proteins in innate and adaptive immunity[J]. Nature Reviews Immunology, 2002, 2(3): 185–194. DOI:10.1038/nri749.
[39] WAX M B, TEZEL G, YANG J, et al. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated t-cell-derived fas-ligand[J]. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 2008, 28(46): 12085–12096. DOI:10.1523/JNEUROSCI.3200-08.2008.
[40] LUO C, YANG X, KAIN A D, et al. Glaucomatous tissue stress and the regulation of immune response through glial toll-like receptor signaling[J]. Investigative Ophthalmology & Visual Science, 2010, 51(11): 5697–5707. DOI:10.1167/iovs.10-5407.
[41] TSAI T, GROTEGUT P, REINEHR S, et al. Role of heat shock proteins in glaucoma[J]. International Journal of Molecular Sciences, 2019, 20(20): E5160. DOI:10.3390/ijms20205160.
[42] ASEA A, KRAEFT S-K, KURT-JONES E A, et al. HSP70 stimulates cytokine production through a cd14-dependant pathway, demonstrating its dual role as a chaperone and cytokine[J]. Nature Medicine, 2000, 6(4): 435–442. DOI:10.1038/74697.
[43] LAMB J R, YOUNG D B. T cell recognition of stress proteins. a link between infectious and autoimmune disease[J]. Molecular Biology & Medicine, 1990, 7(4): 311–321.
[44] CASOLA C, SCHIWEK J E, REINEHR S, et al. S100 alone has the same destructive effect on retinal ganglion cells as in combination with hsp 27 in an autoimmune glaucoma model[J]. Journal of Molecular Neuroscience: MN, 2015, 56(1): 228–236. DOI:10.1007/s12031-014-0485-2.
[45] WEST E E, KOLEV M, KEMPER C. Complement and the regulation of t cell responses[J]. Annual Review of Immunology, 2018, 36(1): 309–338. DOI:10.1146/annurev-immunol-042617-053245.
[46] LO M W, WOODRUFF T M. Complement: bridging the innate and adaptive immune systems in sterile inflammation[J]. Journal of Leukocyte Biology, 2020, 108(1): 339–351. DOI:10.1002/JLB.3MIR0220-270R.
[47] S B, S R, HB D, et al. [Complement activation after induction of ocular hypertension in an animal model][J/OL]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft, 2015, 112(1)[2022–09–28]. https://pubmed.ncbi.nlm.nih.gov/24942221/. DOI:10.1007/s00347-014-3100-6.
[48] CHEN J, JIANG C, HUANG Q, et al. Detection of plasma complement and immune globulin in different sorts of glaucoma[J]. European Journal of Ophthalmology, 2022, 32(5): 2907–2912. DOI:10.1177/11206721221074202.
[49] HARDER J M, BRAINE C E, WILLIAMS P A, et al. Early immune responses are independent of rgc dysfunction in glaucoma with complement component c3 being protective[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(19): E3839–E3848. DOI:10.1073/pnas.1608769114.
[50] HUBENS W H G, BECKERS H J M, GORGELS T G M F, et al. Increased ratios of complement factors c3a to c3 in aqueous humor and serum mark glaucoma progression[J]. Experimental Eye Research, 2021, 204: 108460. DOI:10.1016/j.exer.2021.108460.
[51] BOSCO A, ANDERSON S R, BREEN K T, et al. Complement c3-targeted gene therapy restricts onset and progression of neurodegeneration in chronic mouse glaucoma[J]. Molecular Therapy: The Journal of the American Society of Gene Therapy, 2018, 26(10): 2379–2396. DOI:10.1016/j.ymthe.2018.08.017.
[52] KUEHN M H, KIM C Y, OSTOJIC J, et al. Retinal synthesis and deposition of complement components induced by ocular hypertension[J]. Experimental Eye Research, 2006, 83(3): 620–628. DOI:10.1016/j.exer.2006.03.002.
[53] KUEHN M H. Immune phenomena in glaucoma and conformational disorders: why is the second eye not involved?[J]. Journal of Glaucoma, 2014, 23(8 Suppl 1): S59-61. DOI:10.1097/IJG.0000000000000115.
[54] REINEHR S, REINHARD J, GANDEJ M, et al. Simultaneous complement response via lectin pathway in retina and optic nerve in an experimental autoimmune glaucoma model[J]. Frontiers in Cellular Neuroscience, 2016, 10: 140. DOI:10.3389/fncel.2016.00140.
[55] REINEHR S, REINHARD J, GANDEJ M, et al. S100B immunization triggers nfκb and complement activation in an autoimmune glaucoma model[J]. Scientific Reports, 2018, 8(1): 9821. DOI:10.1038/s41598-018-28183-6.
[56] WILLIAMS P A, TRIBBLE J R, PEPPER K W, et al. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma[J]. Molecular Neurodegeneration, 2016, 11: 26. DOI:10.1186/s13024-016-0091-6.
[57] REINEHR S, GOMES S C, GASSEL C J, et al. Intravitreal therapy against the complement factor c5 prevents retinal degeneration in an experimental autoimmune glaucoma model[J]. Frontiers in Pharmacology, 2019, 10: 1381. DOI:10.3389/fphar.2019.01381.
[58] SUNIL S ADAV, JIN WEI, YAP TERENCE, et al. Proteomic analysis of aqueous humor from primary open angle glaucoma patients on drug treatment revealed altered complement activation cascade[J/OL]. Journal of proteome Research, 2018, 17(7)[2022–09–28]. https://pubmed.ncbi.nlm.nih.gov/29901396/. DOI:10.1021/acs.jproteome.8b00244.
[59] VERNAZZA S, TIRENDI S, BASSI A M, et al. Neuroinflammation in primary open-angle glaucoma[J]. Journal of Clinical Medicine, 2020, 9(10): E3172. DOI:10.3390/jcm9103172.
[60] TEZEL G. The role of glia, mitochondria, and the immune system in glaucoma[J]. Investigative Opthalmology & Visual Science, 2009, 50(3): 1001. DOI:10.1167/iovs.08-2717.
[61] BLOCK M L, ZECCA L, HONG J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms[J]. Nature Reviews. Neuroscience, 2007, 8(1): 57–69. DOI:10.1038/nrn2038.
[62] CHONG R S, MARTIN K R. Glial cell interactions and glaucoma[J]. Current Opinion in Ophthalmology, 2015, 26(2): 73–77. DOI:10.1097/ICU.0000000000000125.
[63] WEI X, CHO K-S, THEE E F, et al. Neuroinflammation and microglia in glaucoma: time for a paradigm shift[J]. Journal of Neuroscience Research, 2019, 97(1): 70–76. DOI:10.1002/jnr.24256.
[64] GRAMLICH O W, TEISTER J, NEUMANN M, et al. Immune response after intermittent minimally invasive intraocular pressure elevations in an experimental animal model of glaucoma[J]. Journal of Neuroinflammation, 2016, 13(1): 82. DOI:10.1186/s12974-016-0542-6.
[65] NORISTANI R, KUEHN S, STUTE G, et al. Retinal and optic nerve damage is associated with early glial responses in an experimental autoimmune glaucoma model[J]. Journal of Molecular Neuroscience: MN, 2016, 58(4): 470–482. DOI:10.1007/s12031-015-0707-2.
[66] CASOLA C, REINEHR S, KUEHN S, et al. Specific inner retinal layer cell damage in an autoimmune glaucoma model is induced by gdnf with or without hsp27[J]. Investigative Ophthalmology & Visual Science, 2016, 57(8): 3626–3639. DOI:10.1167/iovs.15-18999R2.
[67] KUEHN S, GROTEGUT P, SMIT A, et al. Important role of microglia in a novel s100b based retina degeneration model[J]. 2018, 59(9): 4500–4500. .
[68] RODRIGUES-NEVES A C, AIRES I D, VINDEIRINHO J, et al. Elevated pressure changes the purinergic system of microglial cells[J]. Frontiers in Pharmacology, 2018, 9: 16. DOI:10.3389/fphar.2018.00016.
[69] NARAYAN D S, CASSON R J, EBNETER A, et al. Immune priming and experimental glaucoma: the effect of prior systemic lipopolysaccharide challenge on tissue outcomes after optic nerve injury[J]. Clinical & Experimental Ophthalmology, 2014, 42(6): 539–554. DOI:10.1111/ceo.12289.
[70] RAMÍREZ A I, SALAZAR J J, DE HOZ R, et al. Macro- and microglial responses in the fellow eyes contralateral to glaucomatous eyes[M/OL]//Progress in Brain Research. Elsevier, 2015: 155–172[2022–09–29]. https://linkinghub.elsevier.com/retrieve/pii/S0079612315000746. DOI:10.1016/bs.pbr.2015.05.003.
查看更多
相似思维导图模版
首页
我的文件
我的团队
个人中心