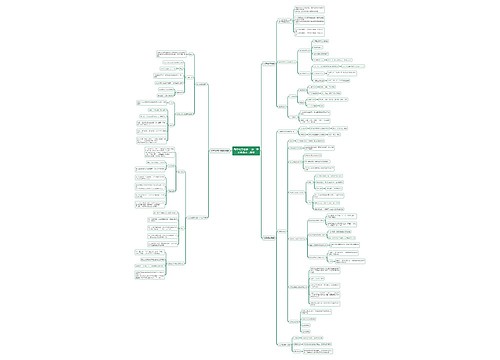
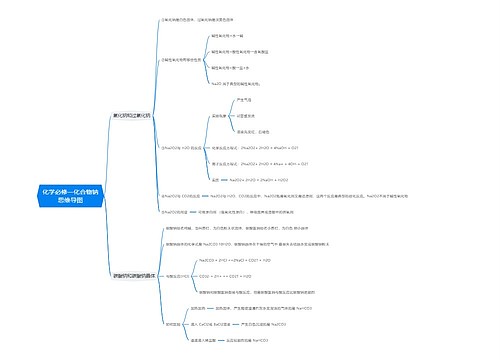
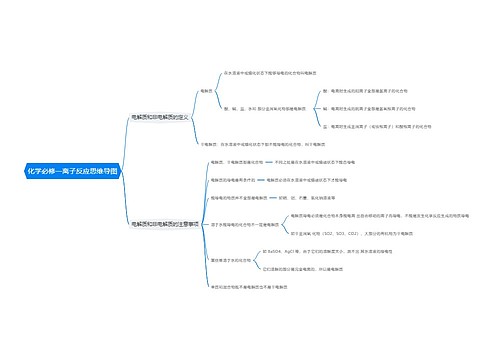
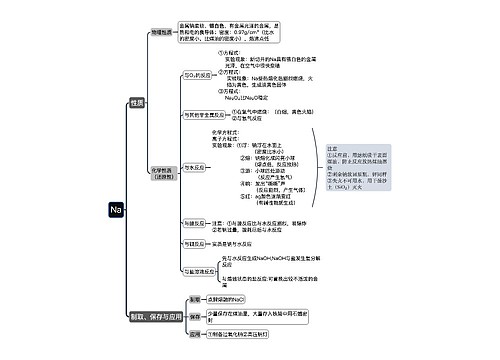
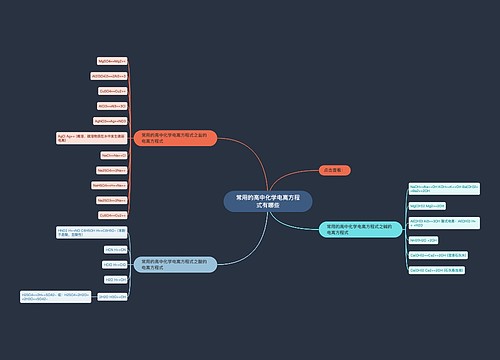
常见的高中化学方程式之热化学方程式思维导图
心不动则不痛
2023-01-13

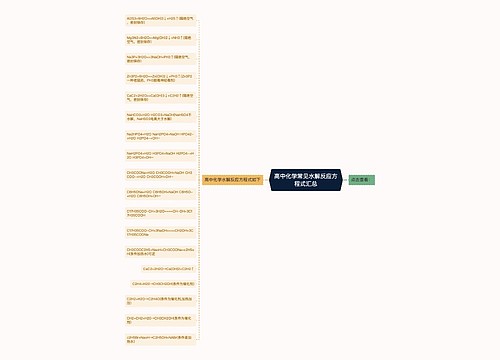
高中化学方程式之热化学方程式是表示化学反应中的物质变化或能量变化,能够表示化学反应热效应的化学方程式叫做热化学方程式。下面是壹壹高考网小编收集整理的常见的高中化学方程式之热化学方程式,供参考。
树图思维导图提供《常见的高中化学方程式之热化学方程式》在线思维导图免费制作,点击“编辑”按钮,可对《常见的高中化学方程式之热化学方程式》进行在线思维导图编辑,本思维导图属于思维导图模板主题,文件编号是:693e9dce0ad43210c8ec668ac015abda
思维导图大纲
相关思维导图模版
904名中国成年人第三磨牙相关知识、态度、行为和病史的横断面调查思维导图
 U633687664
U633687664树图思维导图提供《904名中国成年人第三磨牙相关知识、态度、行为和病史的横断面调查》在线思维导图免费制作,点击“编辑”按钮,可对《904名中国成年人第三磨牙相关知识、态度、行为和病史的横断面调查》进行在线思维导图编辑,本思维导图属于思维导图模板主题,文件编号是:10b9a8a2dd2fb4593f8130ef16c320fc
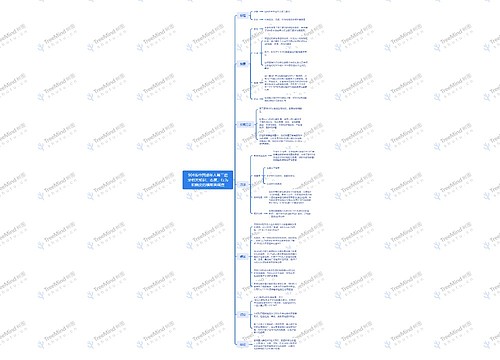
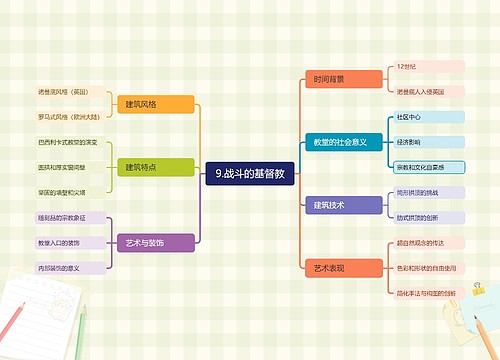
9.战斗的基督教思维导图
 U582679646
U582679646树图思维导图提供《9.战斗的基督教》在线思维导图免费制作,点击“编辑”按钮,可对《9.战斗的基督教》进行在线思维导图编辑,本思维导图属于思维导图模板主题,文件编号是:33d168acd0cd9f767f809c7a5df86e3a