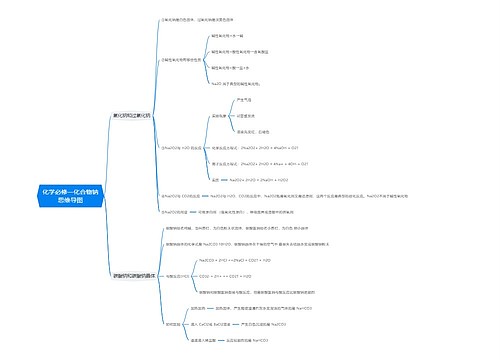
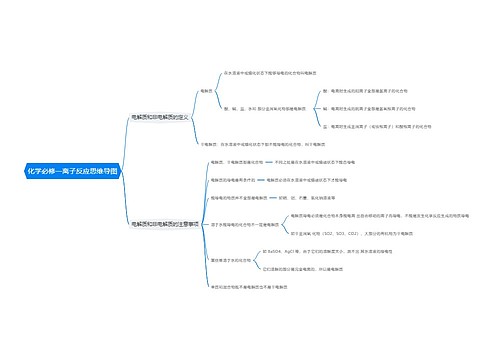
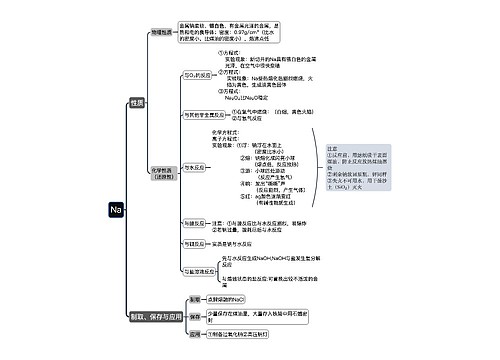
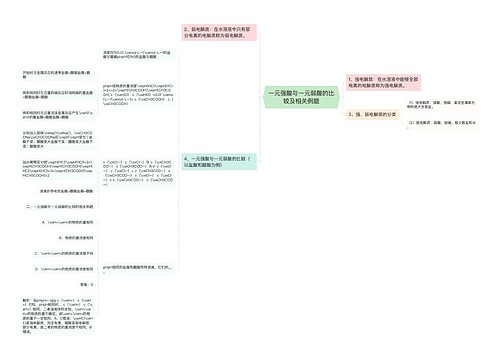
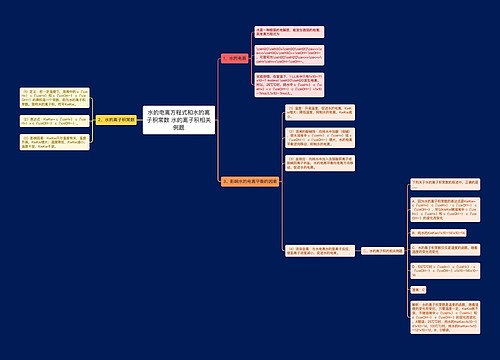
一次电池及原电池的定义 一次电池的相关例题思维导图
心不动则不痛
2023-01-13

一、一次电池及原电池的定义
树图思维导图提供《一次电池及原电池的定义 一次电池的相关例题》在线思维导图免费制作,点击“编辑”按钮,可对《一次电池及原电池的定义 一次电池的相关例题》进行在线思维导图编辑,本思维导图属于思维导图模板主题,文件编号是:1693193ba0f1c9607dac283b6df9fe5a
思维导图大纲
相关思维导图模版
904名中国成年人第三磨牙相关知识、态度、行为和病史的横断面调查思维导图
 U633687664
U633687664树图思维导图提供《904名中国成年人第三磨牙相关知识、态度、行为和病史的横断面调查》在线思维导图免费制作,点击“编辑”按钮,可对《904名中国成年人第三磨牙相关知识、态度、行为和病史的横断面调查》进行在线思维导图编辑,本思维导图属于思维导图模板主题,文件编号是:10b9a8a2dd2fb4593f8130ef16c320fc
9.战斗的基督教思维导图
 U582679646
U582679646树图思维导图提供《9.战斗的基督教》在线思维导图免费制作,点击“编辑”按钮,可对《9.战斗的基督教》进行在线思维导图编辑,本思维导图属于思维导图模板主题,文件编号是:33d168acd0cd9f767f809c7a5df86e3a